"Our technology amplifies the performance of existing vaccines while providing a platform for entirely new ones, from influenza to mRNA and cancer"
Context & Opportunity
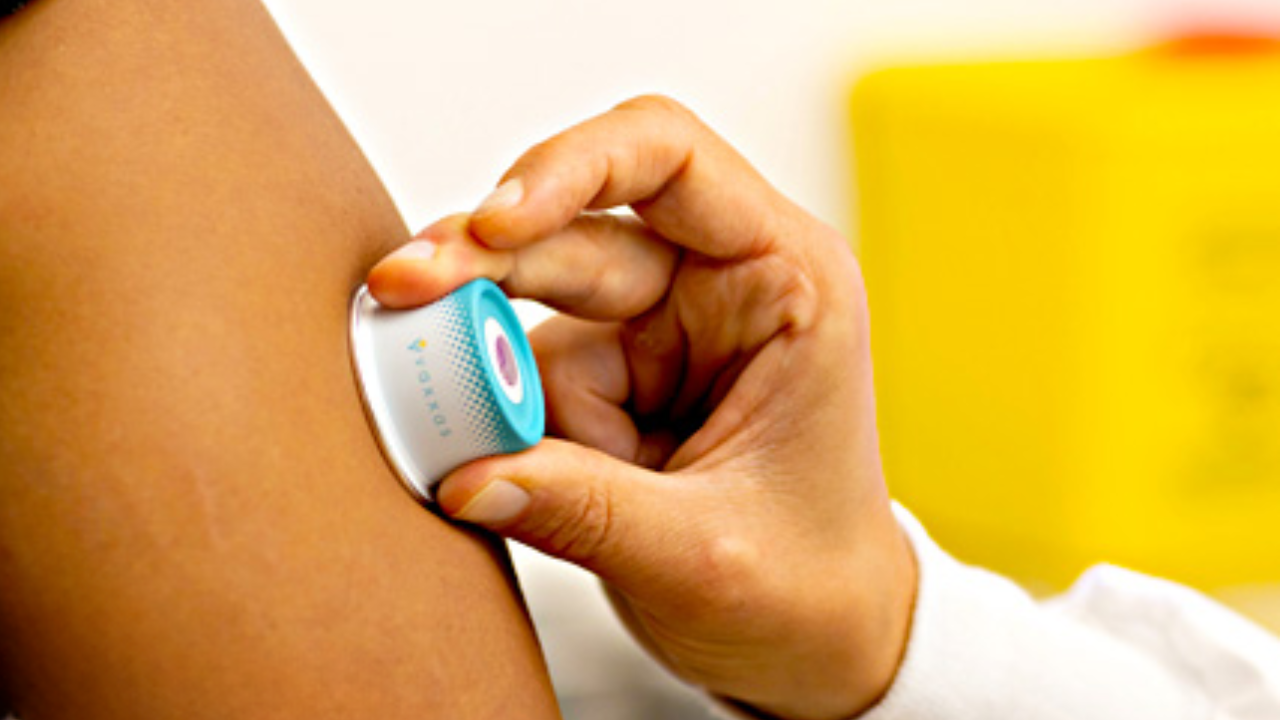
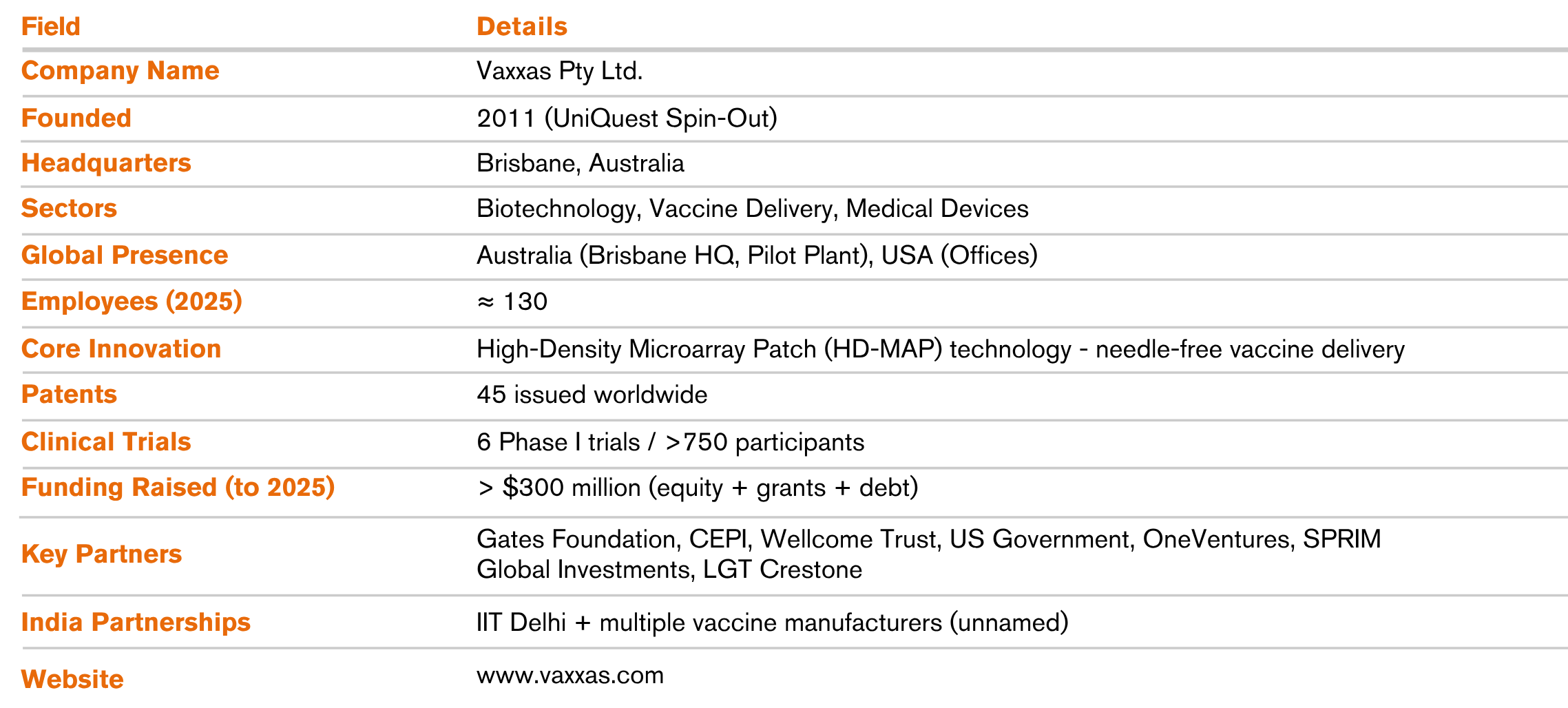
Founded in 2011 as a spin-out from the University of Queensland, Vaxxas is solving a century-old global challenge, how to deliver vaccines more effectively and equitably. The company’s breakthrough High-Density Micro Array Patch (HD-MAP) technology is set to replace needle and syringe with a small patch embedded with thousands of microscopic projections that deliver dry-coated vaccines directly beneath the skin, reducing the need for cold storage, syringes, or highly skilled medical staff.
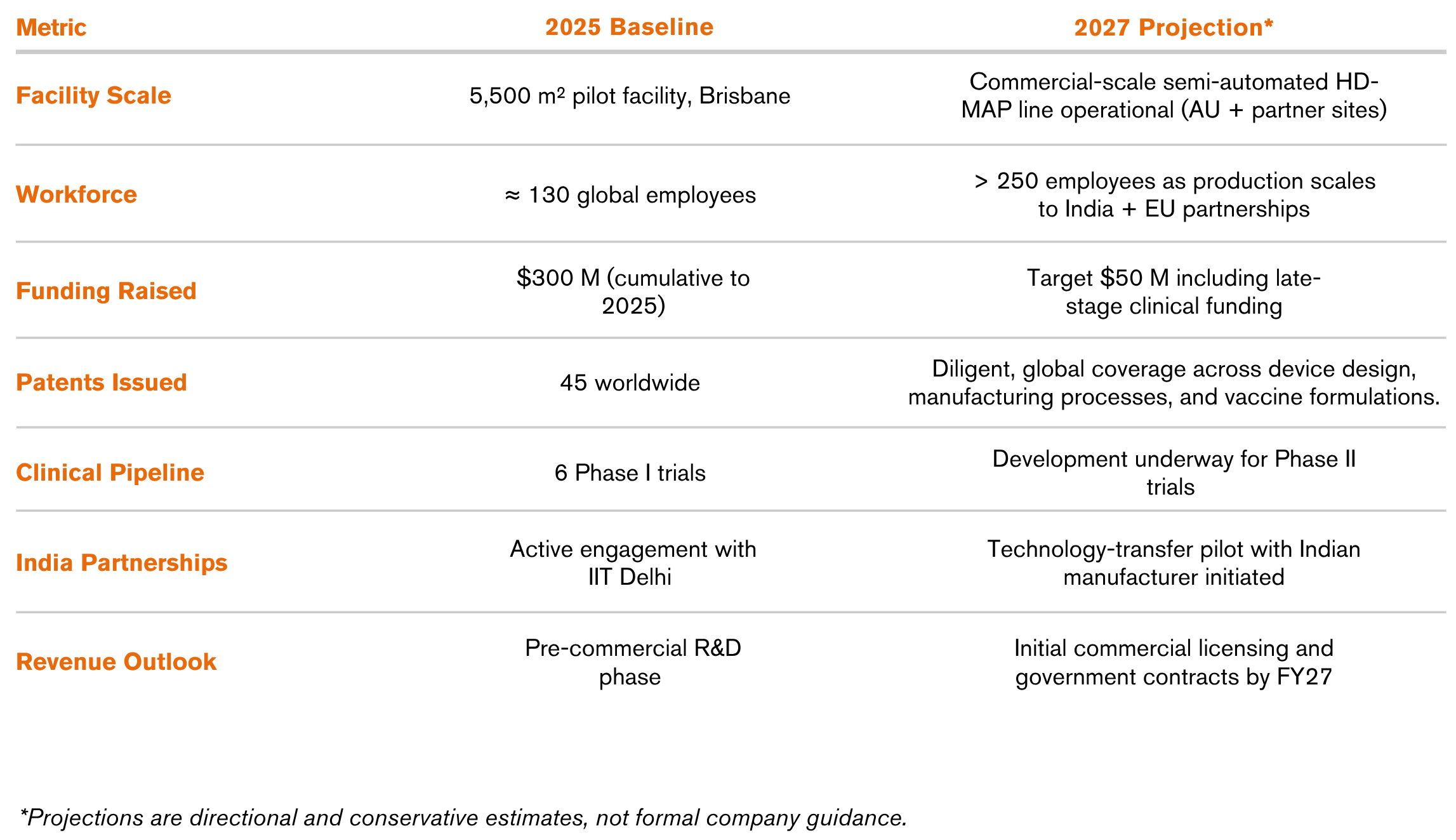
In 2025, Vaxxas has grown into a 130-person biotechnology company headquartered in Brisbane, operating a 5,500 m² pilot-scale manufacturing facility along with offices in the United States.
The company’s HD-MAP technology addresses one of the most acute inequities in global health: access. In India, for instance, 52% of the country’s 29,000 vaccine cold-chain points are concentrated in just six states, leaving vast populations underserved. Vaxxas’ thermostable, needle-free delivery platform directly targets this imbalance by enabling vaccines to be stored, transported, and administered at elevated temperatures, reducing wastage and expanding reach into remote regions.
"Our HD-MAP platform has the potential to redefine global vaccination, by offering a stable, easy-to-use solution that can be deployed almost anywhere.
- Michael Junger, Director of Industry & Government Relations, Vaxxas
With India producing over 60% of the world’s vaccines, Vaxxas’ technology offers a complementary solution - Australian innovation meeting Indian scale. The firm’s dry-coated HD-MAP platform is also opening frontiers in mRNA and cancer vaccine delivery, aligning with the global shift towards next-generation biologics. As climate change drives rising mosquito-borne diseases, Vaxxas’ technology also represents a scalable technology response to new and emerging global health risks.

Strategy & Execution
Unlike traditional vaccine manufacturers, Vaxxas operates a platform business designed for collaboration, not competition. Its model enables three modes of engagement:
- Supplying complete HD-MAP devices for final coating by pharmaceutical partners;
- Providing technology transfer for end-to-end manufacturing; and
- Licensing HD-MAP technology directly to governments and pharmaceutical firms.
This hybrid model ensures flexibility across partner capabilities while protecting Vaxxas’ vast intellectual property portfolio which currently includes 45 granted patents covering device design, manufacturing processes, and vaccine formulations.
"Vaxxas offers an opportunity for manufacturers to build or use its devices and aseptic coating technology to complement their existing capabilities.
Vaxxas recognises India as both a strategic manufacturing hub and a testbed for scalable vaccine delivery. The company is currently exploring collaborative projects with IIT Delhi, focused on storage, delivery, pricing, and packaging solutions tailored for Indian conditions. These partnerships also function as gateways to government networks and industrial alliances, advancing localisation and policy alignment simultaneously.
Vaxxas’ diverse workforce comprising professionals from across all continents, many recruited from Queensland’s global universities such as UQ, Griffith, and QUT, strengthens the company’s cross-cultural agility and ability to conduct business with companies around the world.
Vaxxas’ HD-MAP platform has completed six Phase I clinical trials involving over 750 participants, including vaccines for COVID-19, influenza, and measles/rubella. Results show up to sixfold dose reduction with equivalent and superior immune responses compared to conventional needle delivery.
The company’s printhead-based aseptic coating system enables rapid, high-precision manufacturing at commercial scale, fully compliant with clinical standards. To continue its scale towards global readiness, Vaxxas recently secured $90 million (Series D + debt) bringing the company’s lifetime funding to above $300 million, supported by global health leaders such as the Gates Foundation, CEPI, Wellcome Trust, US Government, OneVentures, SPRIM Global Investments and LGT Crestone.
Vaxxas Chair Sarah Meibusch noted:
"This result underscores the confidence that leading investors have in Vaxxas’ disruptive technology and the progress the team has made toward scaling up and commercialisation.
The funding underwrites installation of semi-automated manufacturing lines and late-stage clinical trials, providing a runway through to 2027.
Impact & Results

Vaxxas has achieved demonstrable progress in both technology performance and partnership traction:
- Facility Scale: 5,500 m² pilot plant in Brisbane
- Workforce: ~130 employees (Australia + US)
- Patent Portfolio: 45 issued patents
- Clinical Trials: 6 Phase I studies / 750 participants
- Dose Efficiency: Up to 6× vaccine-sparing effect
- India Engagement: Collaborations with IIT Delhi + vaccine manufacturers
- Funding Raised: > $300 million (cumulative, equity + grants + debt)
The HD-MAP technology is designed to directly address systemic weaknesses in the vaccine supply chain, cold-chain fragility, healthcare-worker shortages, and last-mile access. Its potential to transform stockpiling and rapid-response vaccination is recognised by governments and donors worldwide.
"India’s vaccine strength and Australia’s biotech ingenuity are deeply complementary

Lessons & Insights
Market Entry & Execution
Vaxxas illustrates how a SME can internationalise through partnerships and technology transfer rather than physical expansion. Its adaptive engagement models minimise capital intensity while building strategic interdependence with local players.
Core Takeaway
In healthcare markets where scale and regulation are barriers, platform partnerships can offer faster and more secure routes to impact.
Cultural Fluency & Workforce Diversity
A diverse team provides a decisive enabler for collaboration with global markets building internal resilience and global credibility.
Core Takeaway
Cross-cultural teams are an innovation advantage.
Regulatory & Policy Alignment
While trade frameworks like AI-ECTA ease movement of inputs and IP, regulatory synchronisation for biologics remains complex. Vaxxas’ reliance on academic and government partnerships provides the long-term route to trust, certification and adoption.
Core Takeaway
In life sciences, policy engagement must parallel technology development.
Operational Discipline
Vaxxas’ tight capital governance, self-developed strategy, executive leadership, and phased automation approach, underscores the importance of financial discipline and IP protection in biotech scale-ups
Core Takeaway
Scalability in biotech depends on the right sequence: prove efficacy, secure IP, then industrialise.
Over the next 3–5 years, Vaxxas plans to deepen partnerships in India, focusing on:
- Technology transfer for localised HD-MAP manufacturing;
- Joint research programs on compatible thermostable vaccines;
- Next-generation applications in mRNA and cancer immunotherapy.
With India’s vaccine sector expanding under Make in India, and Australia’s biotechnology capabilities accelerating under the National Reconstruction Fund, Vaxxas sits at the intersection of two complementary strengths, India’s scale and Australia’s science. Its vision is clear: to make patch-based immunisation the global standard and eliminate needles from routine vaccination altogether.
"Vaxxas shows that true innovation is not just about new technology, it’s about solving access, logistics, and equity challenges at once.
-Michael Junger, Vaxxas
All information has been verified from primary company submissions, official filings, interview transcripts, and secondary materials cited in the References section.

Company Snapshot
KPI Impact Snapshot